Online roundtable ``Development of clinical trials in Romania during the COVID 19 pandemic period``
Schedule
Find out first about the next roundtable date!
Login / register to subscribe![]() Post-event edit
Post-event edit
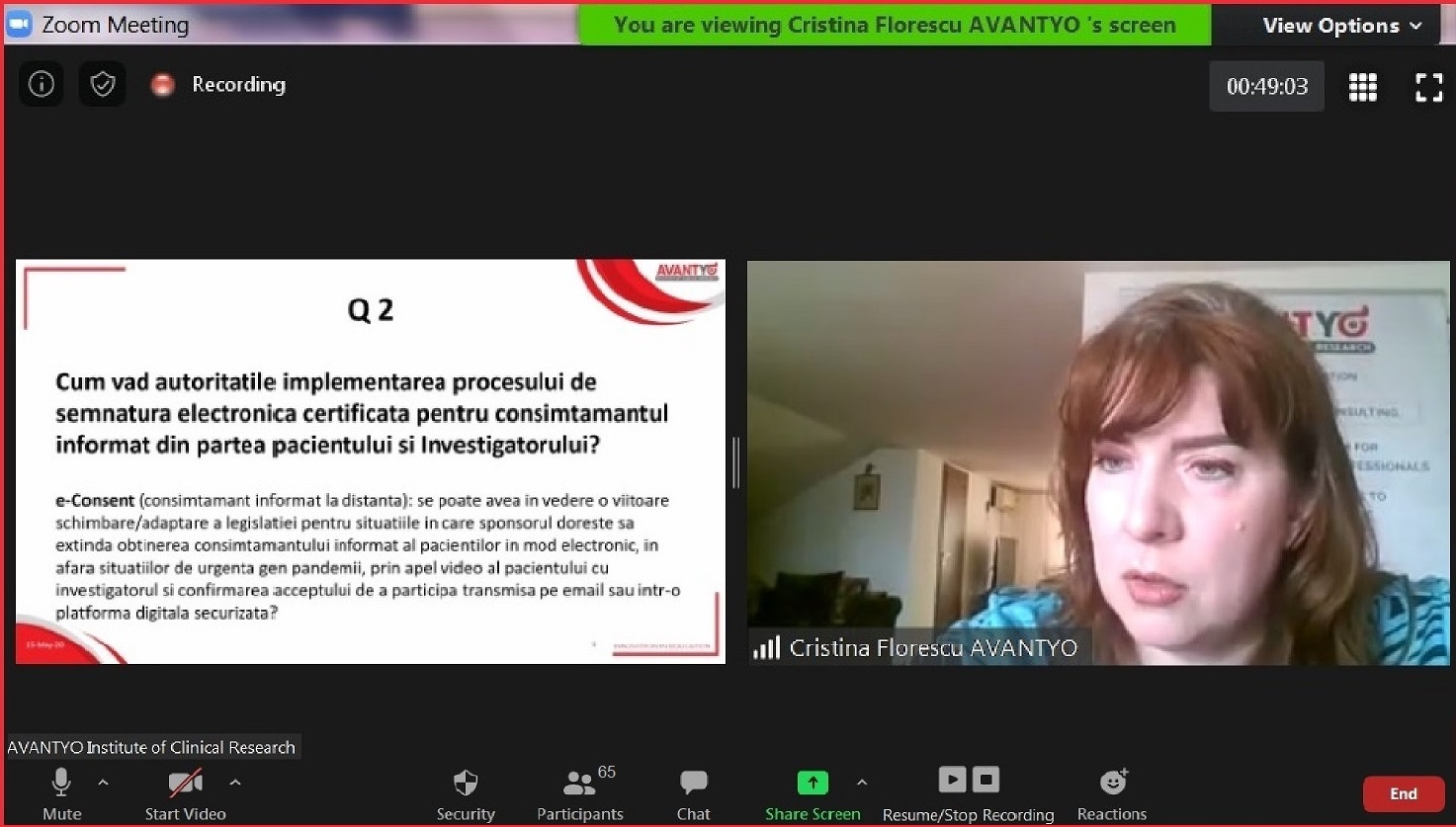
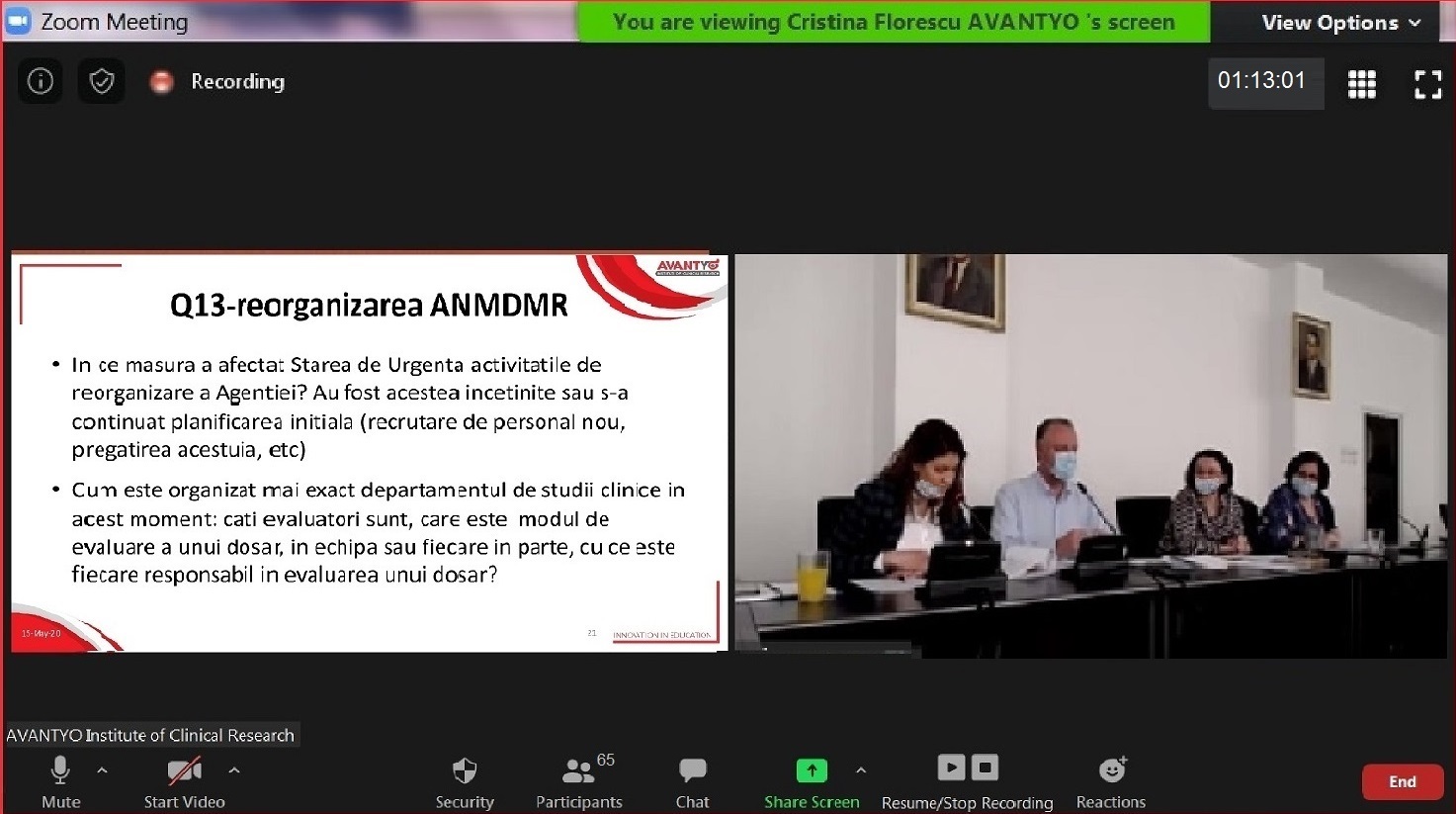
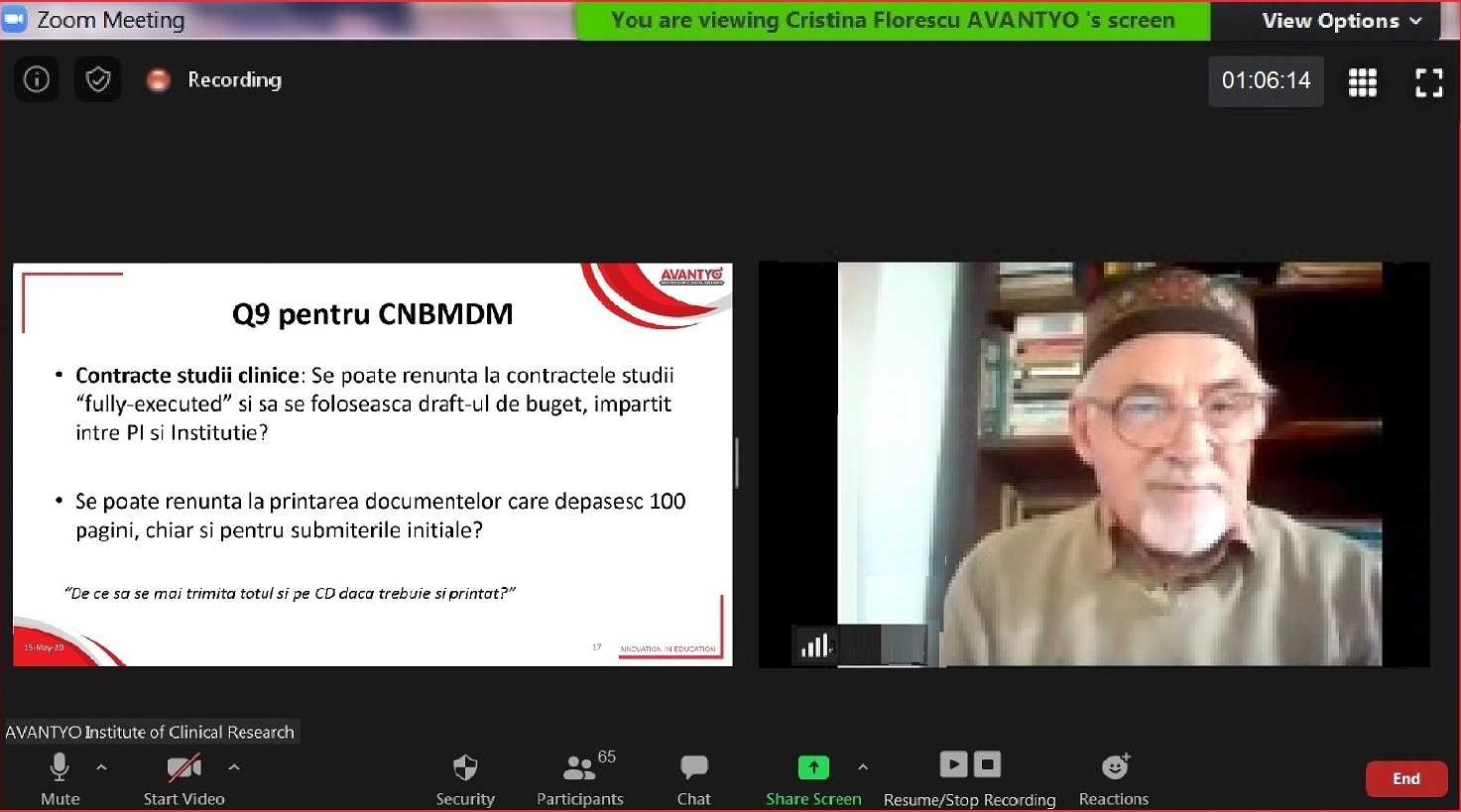
Click here for the Minute of the Meeting.
.
 Find out directly from the Romanian competent authorities, the National Agency for Medicines and Medical Devices in Romania (NADMDR) and the National Commission for Bioethics of Medicines and Medical Devices the newest information regarding conducting the research studies during the COVID-19 pandemic that affects the deployment of clinical trials in Romania.
Find out directly from the Romanian competent authorities, the National Agency for Medicines and Medical Devices in Romania (NADMDR) and the National Commission for Bioethics of Medicines and Medical Devices the newest information regarding conducting the research studies during the COVID-19 pandemic that affects the deployment of clinical trials in Romania.
Register for free to this online roundtable held on Friday, May 15, 2020!
.
Context
Restrictions on movements and stay-at-home orders have helped to mitigate the spread of COVID-19 in Romania. However, these successful strategies have raised obstacles in clinical trials that are essential to finding effective treatments for many other important diseases.
All over the world, the dramatic declines during this pandemic indicate that clinical trials are particularly vulnerable to the state of public health.
There is an urgent need of answers helping to ensure the continuity of existing trials and to start new studies in Romania as part of the worldwide effort for the humans' health.
AVANTYO Institute of Clinical Research has taken the initiative to organize the roundtable “Development of clinical trials in Romania during the COVID 19 pandemic period" with the purpose to bring all together around one table the representatives of the competent authorities and the pharmaceutical companies & contract research organizations.
If you are involved in monitoring or regulatory affairs for clinical trials and you work in a CRO or Pharma Company, this event is the right place to raise your questions.
Registration is open until Thursday, 14th of May. The number of participants is limited.
Join us and register now! Check the right side of this page and make your reservation!
Roundtable Details:
Date/Time: Friday, May 15 at 12:00 PM, Bucharest time (GMT+3)
Location: online. After the registration, you will be provided with the ZOOM meeting details.
Language: Romanian
Host:
 .
.
Dr Cristina Florescu Moraid, CEO of AVANTYO Institute of Clinical Research
.
..
Special Guests:
 .
.
Dr Roxana Stroe – President of the National Agency for Drugs and Medical Devices Romania (NADMDR).
.
.
 .
.
Dr Bujor Eugen Almasan – Vice-President of the National Agency for Drugs and Medical Devices Romania (NADMDR)
..
.
 .
.
Dr Mirela Vita – Lead of Clinical Trials Department at the Romanian National Agency for Drugs and Medical Devices Romania (NADMDR)
..
.
 .
.
Dr Adina Pirvu - GCP Inspector at Romanian National Agency for Drugs and Medical Devices Romania (NADMDR).
.
..
 .
.
Prof. Dr Constantin Mircioiu – Secretary of the National Ethics Commission, from Romania
.
..
Participants' profile: specialists in monitoring and regulatory affairs for clinical trials, from CROs or Pharma Companies / Sponsors.
Seats: up to 75 attendants. Deadline for registration: May 14, 2020, 17:00
Timing:
11:45 – 12:00 Check-in
12:00 – 13:00 Roundtable
Note:
Attendance only by online reservation. Booking the seat si available after registering on this site (MyAccount).
The event will be held on ZOOM and it will be recorded. The minute of this meeting will be published on this site.
After this event, you will be asked to fill in an online anonymous questionnaire.
During the online meeting, the participants’ names will be disclosed to the representatives of the competent authorities. Also, the list with the name of companies participating in this roundtable will be shared to the competent authorities, before the event.
Register now to receive your invitation! Check the right side of this page and make your reservation!
![]()
Post-event edit
Click here for the Minute of the Meeting.
.
.
For assistance please contact us at info@avantyo.com and diana.lupu@avantyo.com, 0726 840 456
The professionals interested to attend the next roundtable with the Romanian competent authorities, about clinical trials deployment in Romania, are invited to send us an email.
In order to be always informed about AVANTYO courses and events, please subscribe to our newsletter.

For previous events, please access one of the following links.